Geoduck Juvenile DNA Extractions
Extracted DNA from Juvenile Geoduck Tissue previously homogenized using the Sample Homogenization Protocol
Sample list
- EPI-167
- EPI-168
- EPI-175
- EPI-176
- EPI-187
- EPI-188
- EPI-205
- EPI-206
- EPI-220
- EPI-221
- EPI-229
- EPI-230
DNA Extractions
- added ~20mg of tissue to 180µl of Buffer ATL and 20µl of Proteinase K
- Proceeded with DNA Extraction Protocol
- Did not do the RNase treatment
- Eluted in 200µl of AE Buffer
DNA Quantification
- Used 1µl of sample and 199µl of Qubit Mix
- Ran Qubit dsDNA BR DNA Quantification Protocol
- Saved samples at -80°C in 2 aliquots (6µl,~190µl)
DNA Concentrations
| Sample.ID | Qubit Conc(ng/µl) | Dilution | Initial Conc(ng/µl) |
|---|---|---|---|
| EPI-230 | 10.1 | 1 | 10.1 |
| EPI-229 | 17.4 | 1 | 17.4 |
| EPI-221 | 30.0 | 1 | 30.0 |
| EPI-220 | 48.8 | 1 | 48.8 |
| EPI-206 | 18.1 | 1 | 18.1 |
| EPI-205 | 11.9 | 1 | 11.9 |
| EPI-188 | 14.5 | 1 | 14.5 |
| EPI-187 | 17.1 | 1 | 17.1 |
| EPI-176 | 7.8 | 1 | 7.8 |
| EPI-175 | 19.6 | 1 | 19.6 |
| EPI-168 | 6.2 | 1 | 6.2 |
| EPI-167 | 13.2 | 1 | 13.2 |
- a 190µl aliquot with an average of ~17.5ng/µl has ~3µg of DNA
- need to run gel quality check of DNA
DNA Quality 20161003
Ran a quality check of DNA using a 1% TAE gel in 1x TAE running buffer
Gel Preparation
TAE
- 40 mM Tris (pH 7.6)
- 20 mM acetic acid
- 1 mM EDTA
Samples
###Gel 1
- O Gene Ruler Mix SM 1173 Thermo Fisher 0.1µg/µl Range 100–10,000 bp
- Test 1
- Test 2
- EPI-167
- EPI-168
- EPI-175
- EPI-176
- EPI-187
- EPI-188
- EPI-205
- EPI-206
- O Gene Ruler Mix SM 1173 Thermo Fisher 0.1µg/µl Range 100–10,000 bp
###Gel 2
- O Gene Ruler Mix SM 1173 Thermo Fisher 0.1µg/µl Range 100–10,000 bp
- EPI-220
- EPI-221
- EPI-229
- EPI-230
- O Gene Ruler Mix SM 1173 Thermo Fisher 0.1µg/µl Range 100–10,000 bp
Gel Setup
- Added 0.75g of Agarose to 75ml of TAE and heated until clear
- Added 6µl of Ethidium Bromide to gel
- Poured gel with 12 upper wells and 8 lower wells
- Once gel was set, Added 6µl of 6x loading dye to each sample of 6µl of DNA (6x purple loading dye #B70245 New Endland BioLabs)
- Added 5µl of the sample-loading dye mix to each well
- Ran gel at 100v for 45 minutes
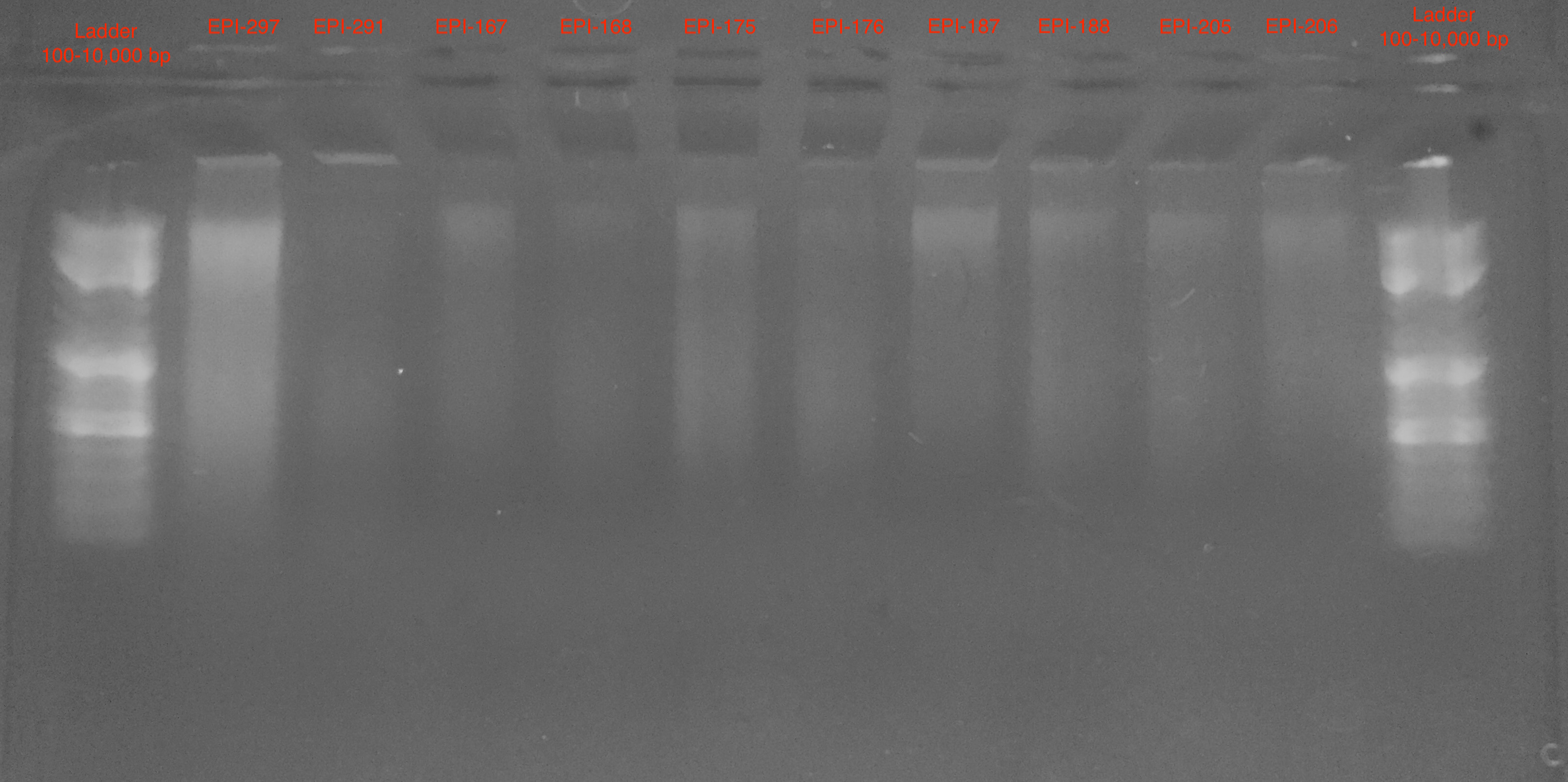
Gel 1
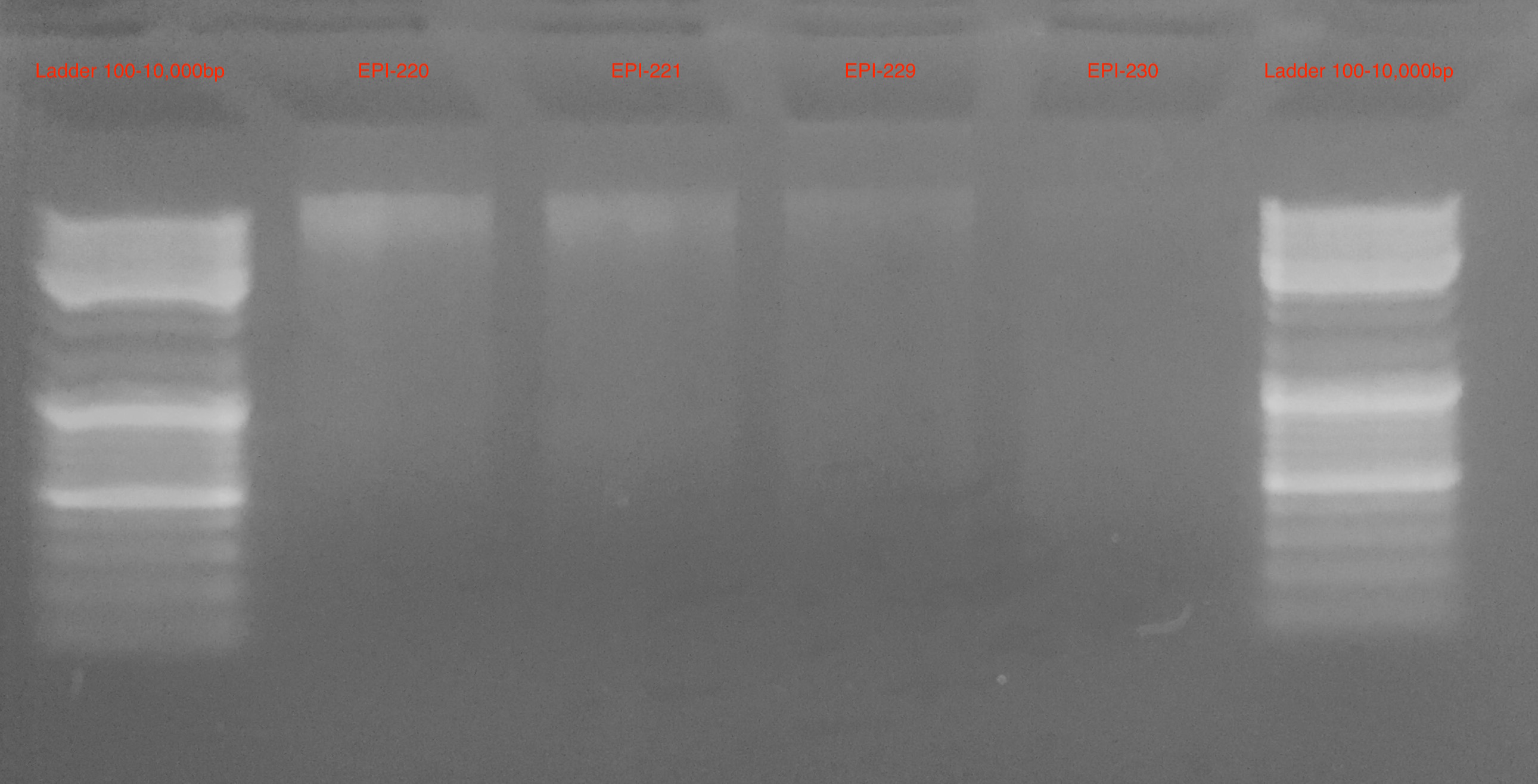
Gel 2
Conclusions
- Samples are more difficult to see in Gel 1
- Appears to be large band >10,000 bp and some smear below
- Samples in Gel 2 are more clear and appear to have relatively discrete bands with minimial smear
-
The data from Gel 2 suggest a good quality DNA extraction with minimial DNA degradation or contamination
- Will move forward with DNeasy DNA Extraction Protocol
Written on September 30, 2016
